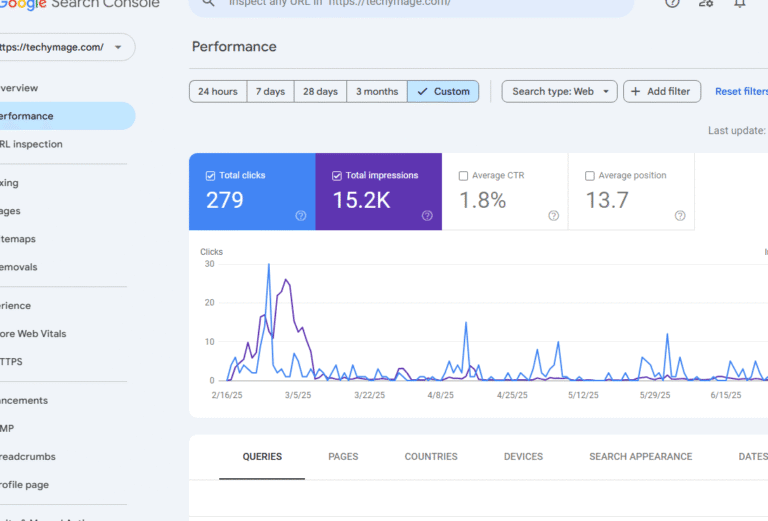
What is HCOOCH CH2 H2O?
The chemical expression HCOOCH + CH2 + H2O refers to a reaction involving methyl formate (HCOOCH₃), methylene groups (CH₂), and water (H₂O). This type of reaction is typically encountered in organic chemistry contexts, especially in mechanisms involving ester hydrolysis, nucleophilic substitution, or radical chemistry.
At its core:
- HCOOCH₃ is methyl formate, a formate ester.
- CH₂ can represent a methylene bridge or act as an intermediate.
- H₂O typically plays a role as a solvent or reactant.
Understanding the interaction between these components can help students and professionals grasp key principles in reaction pathways, organic synthesis, and industrial applications.
Step-by-Step Reaction Mechanism
Initial Reactants and Conditions
In the typical mechanism, we assume:
- HCOOCH₃ acts as the ester compound
- CH₂ is part of an active reagent (often generated in situ)
- H₂O can serve to hydrolyze the ester, releasing formic acid and methanol
Reaction Pathway & Intermediates
The simplified hydrolysis mechanism goes like this:
- Nucleophilic attack of water on the carbonyl carbon of HCOOCH₃
- Formation of a tetrahedral intermediate
- Breakage of the ester bond
- Yielding formic acid (HCOOH) and methanol (CH₃OH)
If CH₂ is part of a reactive compound (e.g., diazomethane or another methylene donor), it may:
Insert into C–H or C–O bonds
Lead to alkylation or substitution reactions
Final Products & Energy Considerations
Products may include:
Formic acid
Methanol
CH₂-inserted derivatives (if CH₂ is part of an alkylation reagent)
The reaction typically proceeds under mild acidic or basic conditions and may be exothermic, especially during ester hydrolysis.
Why This Reaction Matters
Industrial Significance
Methyl formate is widely used in the manufacture of formic acid, solvents, and resins.
Hydrolysis reactions like these are essential in chemical manufacturing.
Role in Organic Synthesis
This mechanism illustrates:
Ester cleavage techniques
Reactivity of CH₂ intermediates
Green chemistry methods using water as a benign reagent
Safety and Handling Notes
Methyl formate is flammable and volatile.
Reactions involving CH₂ radicals (e.g., diazomethane) are toxic and explosive.
Use protective equipment and fume hoods in experimental setups.
Applications in Real Life
1. Pharmaceutical Industry
Synthesis of active pharmaceutical ingredients (APIs) where ester groups need to be hydrolyzed or modified.
2. Polymer & Plastic Production
Formic acid derived from methyl formate is used in polymer catalysis.
CH₂ insertion reactions aid in polymer backbone modifications.
3. Green Chemistry & Sustainability
Water-based hydrolysis promotes eco-friendly synthesis.
Avoids toxic reagents and minimizes waste.
Common Misunderstandings
Is CH₂ a Free Radical Here?
Not always. CH₂ can represent:
A methylene group (neutral, part of a structure)
A radical (highly reactive) depending on the context
Is This Reaction Reversible?
Hydrolysis reactions are typically irreversible, especially in aqueous conditions.
Why Is Water Involved?
Water acts as a nucleophile in hydrolysis and facilitates proton transfers in the reaction mechanism.Visual Diagram of the Reaction
Insert a simple reaction scheme like:
If CH₂ is part of a more advanced compound:
Optional: Use an infographic or simple arrow reaction image.
Related Reactions and Variations
HCOOCH₃ + Methanol: Forms dimethyl formate
With Ethanol: Leads to ethyl formate
With Ammonia: Produces formamide derivatives
With Acid Catalysts: Accelerates ester hydrolysis
FAQs
Q1. What is the role of H₂O in the HCOOCH CH₂ H₂O reaction?
It acts as a nucleophile to break ester bonds.
Q2. Can this reaction be used in green chemistry?
Yes — especially when using water as the main solvent/reactant.
Q3. Is this reaction part of standard organic chemistry curriculums?
Yes, ester hydrolysis is a foundational concept in both high school and college-level chemistry.
Conclusion
The HCOOCH CH₂ H₂O reaction, while seemingly complex, showcases essential organic chemistry principles — from ester hydrolysis to nucleophilic substitution and methylation. Whether you’re working in a lab or learning in a classroom, understanding how these molecules interact provides a solid foundation in reaction mechanisms and real-world applications.
If you are interested in a guest post then contact us via email: mudassirali@techymage.com.